Address
No32 Zhongnan Road, Songjiang District, Shanghai,China
Work Hours
Monday to Friday: 9AM - 6PM
Email
sales@laboinst.com
Address
No32 Zhongnan Road, Songjiang District, Shanghai,China
Work Hours
Monday to Friday: 9AM - 6PM
Email
sales@laboinst.com

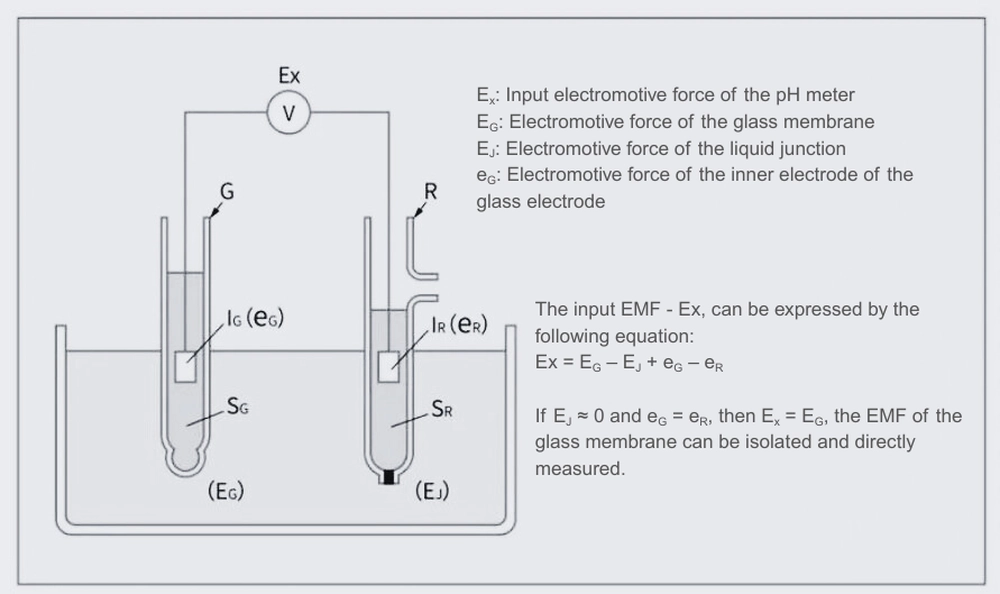
When an electrode with a special glass membrane that reacts with hydrogen ions is immersed in a solution to be measured, an electromotive force (EMF) corresponding to the activity of hydrogen ions is generated on the surface of the glass membrane. This EMF cannot be directly detected in isolation, so a voltmeter should be connected between the glass membrane and the reference electrode, and the EMF is expressed as the potential difference between the voltmeter (pH meter) and the reference electrode.
The index used to represent hydrogen ion activity is called “pH”, which has a logarithmic relationship with hydrogen ion activity: pH = -log[H⁺]. To measure pH, the pH meter is first calibrated using a standard buffer solution of known pH values. This allows the pH value to be directly determined based on the EMF produced by the corresponding hydrogen ion activity.
A pH electrode equipped with a specially formulated glass membrane is called a measurement electrode, while a reference electrode is known as the reference electrode. The reference electrode contains a high-concentration potassium chloride (KCl) solution and features a liquid junction at its tip. The liquid junction is typically made of porous ceramic and is in contact with the test solution. When the reference electrode contacts the test solution through the liquid junction, it generates a stable and constant electromotive force, which serves as a reference for determining the potential difference with the glass electrode.
The theoretical formula for pH measurement is as follows.

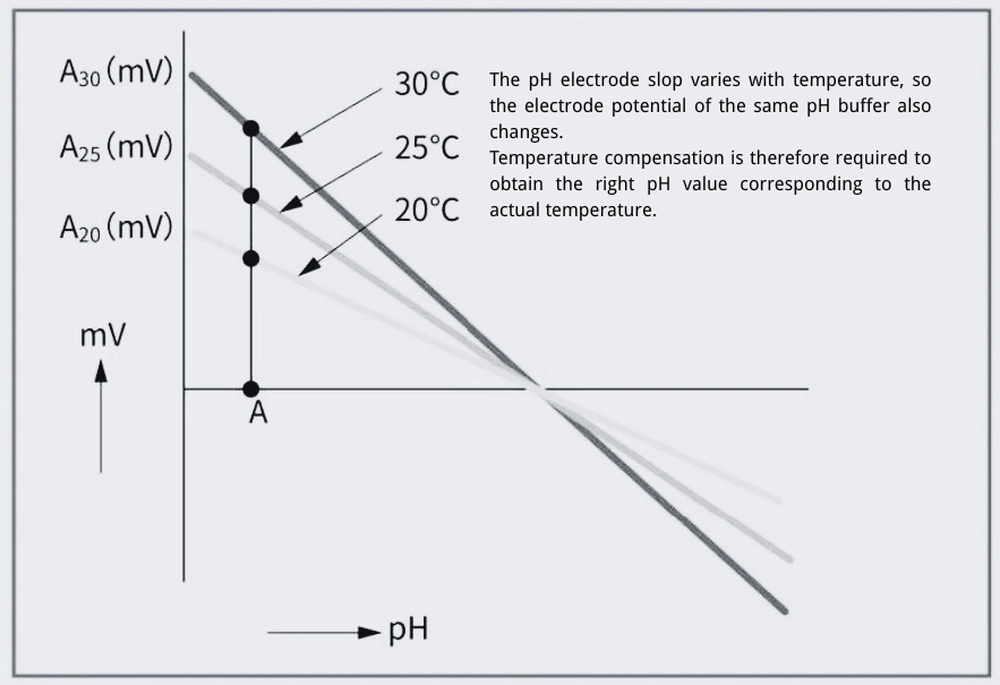
The theoretical slope is 59.16 (at 25°C), representing the potential difference corresponding to a 1-unit change in pH. As shown in the formula, this slope varies with temperature.
To ensure accurate pH measurement, pH meters generally use temperature compensation to correct for the slop changes caused by temperature fluctuations.
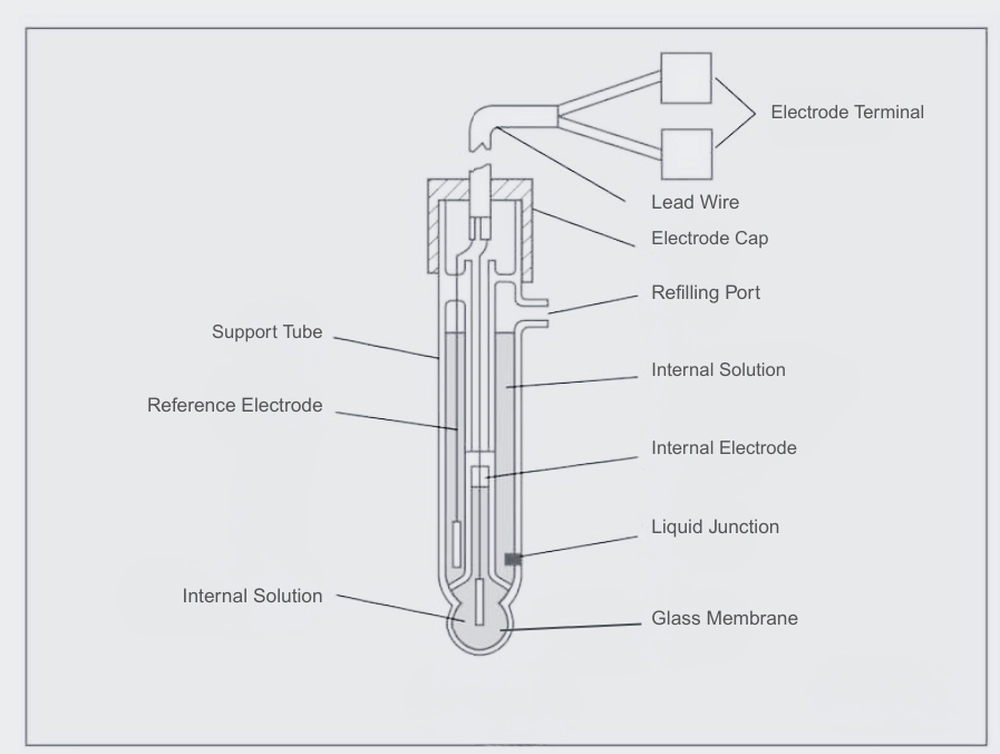
The measuring electrode’s glass tube has a special pH glass membrane at the tip. Inside, it consists of an internal liquid of a defined concentration and an internal electrode that generates a stable electromotive force. The glass membrane is typically a spherical film with a thickness of approximately 0.1 to 0.3 mm. It is easily damaged and requires careful handling. Laboinst’s industrial online pH electrodes adopts a thick-film-treated glass membrane, which offers high resistance to mechanical shock and is an ideal choice for industrial pH monitoring.
To accurately measure pH, only the change in electromotive force across the glass membrane should be detected. Therefore, a reference electrode that maintains a stable electromotive force in any liquid is essential. A Ag/AgCI reference electrode is commonly used, with a 3.3 mol/L KCl solution or saturated KCl solution as the internal solution.
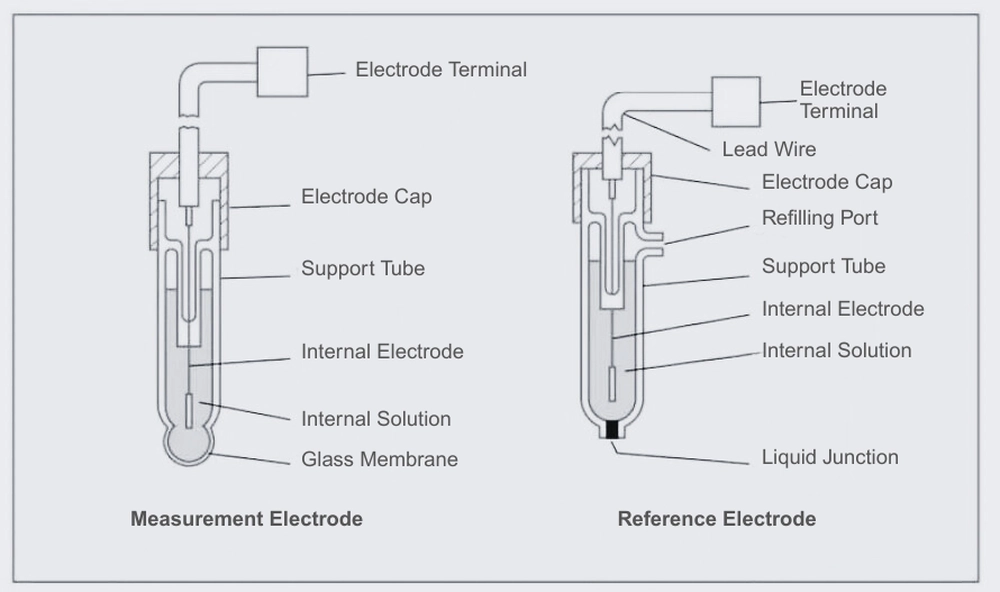
The tip of the reference electrode is equipped with a liquid junction that maintains electrical contact with the test solution. Ideally, the junction should maintain electrical contact while allowing the liquid inside to slowly drain out. The junction is typically made of porous ceramic, though sleeve-type junctions may also be used depending on the application. The contacting surface of the liquid is ground glass.
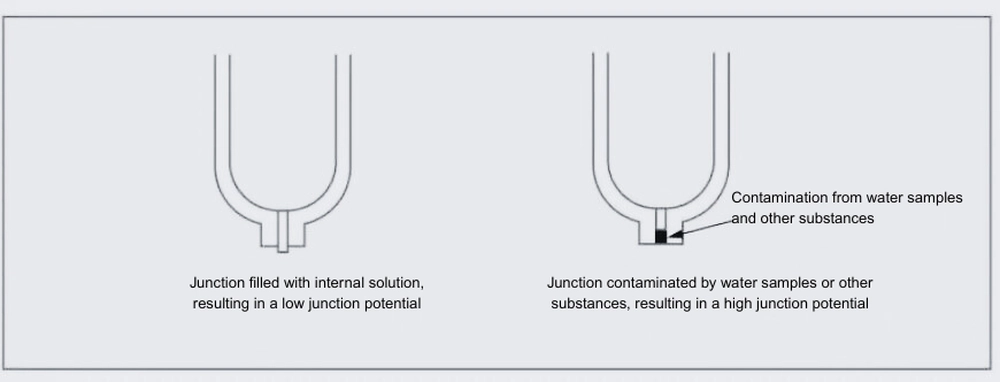
At the liquid junction, liquids flow through tiny channels. Based on electrochemical principles, the difference in mobility between anions and cations passing through them generates a liquid junction potential. Potassium chloride, as mentioned above, generates a very small potential, so it is used as the internal liquid. Upon contact with the sample solution, the sample gradually enters the liquid junction through diffusion. Accumulation of the sample solution at the liquid junction generates a liquid junction potential, causing measurement errors. Increasing the flow rate of the internal solution is an effective way to keep the liquid junction filled with KCl solution. However, increasing the flow rate too much will rapidly deplete the internal solution, causing excessive influx of KCl into the sample solution and potentially affecting pH concentration. Therefore, when selecting liquid junction components, it is important to find conditions that maintain an appropriate flow rate.
The liquid junction potential varies depending on the sample solution and is also affected by factors such as contamination of the liquid junction. Therefore, the potential generated at the liquid junction, encompassing both the theoretical liquid junction potential and potentials caused by contamination, is collectively referred to as the liquid junction potential.
When measuring pH, there is a correlation between pH and electromotive force, with a theoretically fixed slope. For example, at 20°C, the slope is 58.17 mV, at 25°C, it is 59.16 mV, and at 30°C, it is 60.15 mV. Therefore, when measuring pH, it is necessary to measure the temperature and use the slope to correct the slope for each pH change. This process is called temperature compensation. Temperature compensation can be performed manually or automatically using a built-in temperature sensor to determine the theoretical slope based on the temperature. This temperature sensor is called a temperature compensating electrode, and the method of automatically performing temperature compensation based on the measured temperature is called automatic temperature compensation (ATC). Currently, most pH measurements use a built-in temperature sensor for automatic temperature compensation.
pH measurement requires a measurement electrode, a reference electrode, and a temperature sensor for temperature compensation. However, pH combination electrodes that integrate the measurement electrode, reference electrode, and temperature sensor are now common. A typical pH combination electrode features a glass membrane at the tip and a ceramic liquid junction.